What is it about BCAAs – or branched chain amino acids – and leucine, that gets bodybuilders all excited?! Well, it’s got something to do with their role in muscle-building which sets them apart from the other amino acids. Adding BCAA or leucine supplements to your diet can be a great way of helping you to get the most out of your gym time. But, what are BCAAs? How will they help you in your quest for bigger, stronger muscles? Do you even need them? Here’s everything you need to know…
Contents
Essential Amino Acids and Protein Synthesis
Before diving into the science of leucine and BCAAs, let’s look at protein more generally. Protein is a macronutrient, which means it’s a nutrient that the body requires in large amounts. The main dietary macros are carbs, fats, fibre and protein. Protein has numerous roles in the body, one of which is maintaining, repairing and building muscle. Protein molecules vary in structure and length and are made up of simple units known as amino acids.
There are hundreds of different amino acids in nature, but 20 of them are particularly interesting because they are involved in the first stages of protein synthesis, and they’re known as the proteinogenic amino acids. Nine of these 20 are more interesting still because they’re essential to human diets as they can’t be created from other amino acids. These essential amino acids (EAAs) are found in varying proportions in different protein-rich foods and the amounts present are an indication of the food’s protein quality.
Protein synthesis is where certain constituents of the body, such as muscle, skin, enzymes, some hormones, neurotransmitters and immune factors, to name but a few, are created from amino acids. The protein’s original structure is created from the 20 proteinogenic amino acids, with others being incorporated during or after the process. If you don’t consume a good amount of EAA-rich protein, less crucial tissues may be broken down in order to prioritise what the body needs to keep us alive. Because the body prioritises keeping us alive, it sends the amino acids to the immune system and hormones and may steal some from muscle tissue (a bodybuilder’s worst nightmare!). This is why protein malnutrition includes fatigue, muscle wasting, broken skin and a compromised immune system.
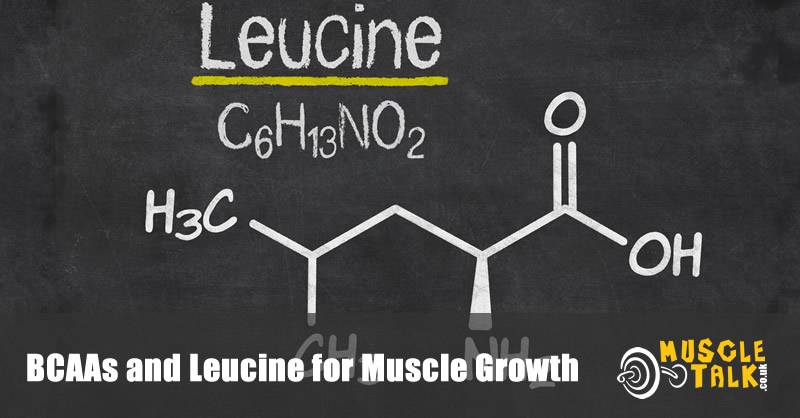
What is Leucine?
Of the nine EAAs, three are known as branched-chain amino acids (BCAAs) because of their structure. These are leucine, isoleucine and valine. BCAAs are present in high concentrations in several tissues including muscle tissue. This partly explains bodybuilders’ and strength athletes’ love for BCAAs. But there’s more to it as BCAAs have other key roles. For example, isoleucine helps to increase the uptake of glucose by cells, which may help to promote energy during exercise [1].
More importantly, however, BCAAs – in particular leucine, which is four times more potent than isoleucine and valine – play a key role in controlling the turning on of protein synthesis. And more protein synthesis leads to bigger muscles!
How Leucine Boosts Muscle Growth
BCAAs stimulate the activity of an enzyme complex called mTOR [2]. mTOR – or “mammalian target of rapamycin” is involved in the regulation of metabolic processes in animal tissues, and this includes protein synthesis for growth, repair and recovery of muscle tissue following exercise [3]. Leucine is particularly key here because, when the concentration of leucine reaches a certain level, the mTOR “switch” is turned on and muscle protein synthesis kicks off.
As BCAAs are abundant in high protein foods, protein synthesis is triggered after a protein-rich meal. It might help to think of mTOR as a switch, which when turned to “on” will initiate protein synthesis with leucine acting as the pressure on the switch. When the level of leucine is high enough, the switch is turned on and muscle tissue can be repaired. When leucine levels go down, the switch is released and protein synthesis turns off.
If mTOR is inactive for long periods, it can lead to muscle wasting, which helps to explain the loss of muscle mass and tiredness in people with advanced wasting diseases, such as the latter stages of AIDS and cancer cachexia.
Benefits of Taking Leucine
As we’ve seen, leucine and BCAA supplements can be incredibly useful to the hard training athlete. The main effects of leucine are its roles in muscle formation and protein synthesis via mTOR activation. These benefits extend to helping to maintain muscle mass whilst following a weight loss regimen, especially when exercise is involved.
Leucine may also help slow down the effects of ageing by helping to preserve muscle mass. Moreover, it’s been shown that leucine supplementation has been associated with a lowering of total and LDL cholesterol levels [4]. This may lead to cardiovascular benefits and a reduced risk of heart disease and stroke.
How Much BCAAs and Leucine Do You Need?
Supplementary leucine has been shown to help increase strength and performance [5], especially with resistance training [6]. However, the effect will depend on how much leucine is present in a meal containing at least 20g of protein [7]. As part of a protein meal, a level of BCAAs totalling at least 4.5g, of which at least 2.2g is leucine [8], has been shown to be sufficient to stimulate protein synthesis.
To achieve this intake, make sure you consume a high-quality protein meal or snack (containing at least 20g protein) or take a BCAA or leucine supplement. Either have around 2.5g of leucine or 5g of a BCAA formula with a 2:1:1 ratio of leucine:isoleucine:valine. Supplementation may be particularly useful in plant-based diets which typically can be a little lower in BCAAs.
When to Take Leucine
Although some people have leucine in their post– or pre-workout shake, the best time to take a leucine supplement is with a meal to boost its leucine content. It might also be useful to have a serving of leucine pre-bedtime to keep mTOR working through the night.
BCAAs and Leucine Key Points
- Protein is made up of amino acids
- There are nine essential amino acids (EAAs)
- Three EAAs are the branched-chain amino acids (BCAAs): leucine, isoleucine and valine
- BCAAs – in particular leucine – trigger the mTOR enzyme and protein synthesis is switched “on”
- An adequate leucine intake helps muscle building and maintenance, and, possibly, cardiovascular health
- High protein meals should containing at least 4.5g BCAA, with 2.2g leucine
- Leucine supplementation may be useful pre-bedtime
References
- Doi M, et al. “Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12 myotubes.” Biochem Biophys Res Commun. 2003; 312(4):1111-7.
- (a) Anthony JC, et al. “Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation.” J Nutr. 2000; 130(2):139-45. (b) Anthony JC, et al. “Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway.” J Nutr. 2000; 130(10):2413-9. (c) Blomstrand E, et al. “Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise”. J Nutr. 2006; 136(1):269s-73s. (d) Drummond MJ, et al. “Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis.” J Physiol. 2009; 587(7):1535-46.
- (a) Kim DH, et al. “mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery.” Cell. 2002; 110(2):163-75. (b) Wipperman MF, et al. “Mammalian Target of Rapamycin: A Metabolic Rheostat for Regulating Adipose Tissue Function and Cardiovascular Health.” Am J Pathol. 2019; 189(3):492-501.
- (a) Zhang Y, et al. “Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms.” Diabetes. 2007; 56:1647-54. (b) Zhao Y, et al. “Leucine supplementation via drinking water reduces atherosclerotic lesions in apoE null mice.” Nature. 2016; 37:196-203.
- (a) Ispoglou T, et al. “Daily L-leucine supplementation in novice trainees during a 12-week weight training program.” Int J Sports Physiol Perform. 2011; 6(1):38-50. (b) Norton LE, et al. “Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise.” J Nutr. 2006; 136(2):533s-7s. (c) Tipton KD, et al. “Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise.” Am J Physiol Endocrinol Metab. 2007; 292(1):E71-6. (d) Tipton KD, et al. “Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise.” Am J Physiol Endocrinol Metab. 2001; 281(2):E197-206.
- (a) Anthony JC, et al. “Leucine Supplementation Enhances Skeletal Muscle Recovery in Rats Following Exercise.” J of Nutrition. 1999; 129(6):1102-6. (b) Layman DK, et al. “Defining meal requirements for protein to optimize metabolic roles of amino acids.” Am J Clin Nutr. 2015; 101(6):1330S-8S.
- Bauer J, et al. “Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group.” J Am Med Dir Assoc. 2013; 14(8):542-59.
- ibid 6(b)
